- Analyzers
- Optics & Sources
- Technologies
- Support
- About
Understanding the Effects of Silicon Contamination
Author Leslie Johnson, Applications Scientist
BACKGROUND
Refiners throughout the petroleum and biofuels industry are taking a closer look at the dangers of silicon in gasoline and ethanol. Silicon is a contaminant and, depending on where the contamination occurs along the supply chain, the repercussions can range from engine problems for gasoline end users, to possible poisoning of naphtha hydrotreater catalysts at the refinery level. This led to ASTM adding a warning about silicon contamination in the Workmanship Section of the gasoline and ethanol specifications:
- Standard Specification for Automotive Spark-Ignition Engine Fuel D4814-19: 6.2.1 Manufacturers and blenders of gasoline and gasoline oxygenate blends shall avoid gasoline blending stocks (for example, purchased used toluene solvents) or denatured fuel ethanol (for example, improperly recycled ethanol) contaminated by silicon containing materials, or both. Silicon contamination of gasoline and gasoline-oxygenate blends has led to fouled vehicle components (for example, spark plugs, exhaust oxygen sensors, catalytic converters) requiring parts replacement and repairs. Test Method D7757 is a procedure for determining silicon content but no specification limits have been established for silicon.1
- Standard Specification for Denatured Fuel Ethanol for Blending with Gasolines for Use as Automotive Spark-Ignition Engine Fuel D4806-19: 6.2.1 Manufacturers, importers, and others denaturing fuel ethanol shall avoid ethanol (for example, improperly recycled ethanol) or denaturants contaminated by silicon-containing materials, or both. Silicon contamination of gasoline oxygenate blends has led to fouled vehicle components (for example, spark plugs, exhaust oxygen sensors, catalytic converters) requiring parts replacement and repairs. Test Method D7757 is a procedure for determining silicon content but no specification limits have been established for this silicon.2
- Standard Specification for Ethanol Fuel Blends for Flexible-Fuel Automotive Spark Ignition Engines D5798-19a: 6.3.1 Manufacturers and blenders of ethanol fuel blends shall avoid ethanol (for example, improperly recycled ethanol), or denaturants and hydrocarbon blend components contaminated by silicon-containing materials, or both. Silicon contamination of gasoline, denatured ethanol, and their blends has led to fouled vehicle components (for example, spark plugs, exhaust oxygen sensors, catalytic converters) requiring parts replacement and repairs. Test Method D7757 is a procedure for determining silicon content but no specification limits have been established for silicon.3

- Standard Specification for Methanol Fuel Blends (M51–M85) for Methanol-Capable Automotive Spark-Ignition Engines D5797-18: 6.2.1 Manufacturers and blenders of methanol fuel blend (M51–M85) shall avoid methanol (for example, improperly recycled methanol), or hydrocarbon blend components contaminated by silicon-containing materials, or both. Silicon contamination of gasoline, denatured ethanol, and their blends has led to fouled vehicle components (for example, spark plugs, exhaust oxygen sensors, catalytic converters) requiring parts replacement and repairs. Test Method D7757 is a procedure for determining silicon that might be applicable to methanol fuel blend (M51–M85). No specification limits have been established for silicon.4
- Standard Specification for Methyl Tertiary-Butyl Ether (MTBE) for Blending With Gasolines for Use as Automotive Spark-Ignition Engine Fuel D5983-18: 5.3 Manufacturers and importers of MTBE shall avoid contamination by silicon-containing materials. Silicon contamination of gasoline-oxygenate blends has led to fouled vehicle components (for example, spark plugs, exhaust oxygen sensors, catalytic converters) requiring parts replacement and repairs. Test Method D7757 is a procedure for determining silicon that might be applicable to MTBE. Additional studies will be needed to include MTBE into the scope of Test Method D7757. No specification limits have been established for silicon.5
ASTM published D7757 Standard Test Method for Silicon in Gasoline and Related Products by Monochromatic Wavelength Dispersive X-ray Fluorescence Spectrometry – paving the way for an ASTM-approved and accurate silicon measurement. This test method falls under the jurisdiction of ASTM D02 on Petroleum Products and Lubricants and is the direct responsibility of Subcommittee D02.3 on Elemental Analysis. Kishore Nadkarni, Ph.D., of Millennium Analytics, Inc., and former chairman of D02.03, notes that D7757 will fill industry needs and that it is already being used successfully for determining silicon concentration in gasoline, gasoline-oxygenate blends, denaturants, and hydrocarbon blend components and denatured fuel ethanol.
CHALLENGE
Silicon contamination of gasoline leads to silica deposits on vehicle components such as spark plugs, catalytic converters, and oxygen sensors. Improper feedback or lack of feedback from failing oxygen sensors can lead to incorrect control of engine air/fuel mixture, which may cause issues such as:
- Rough engine idle, missing, and pinging
- Poor fuel economy
- Increased emissions
- Stalling or not starting
- Catalytic converter damage
Silicon contamination in petroleum refineries may also have a negative impact, including poisoning of naphtha hydrotreater catalysts. When silicon contaminated naphtha is processed in the hydrotreater, silicon compounds are irreversibly adsorbed onto catalyst surfaces. Over time, this results in reduced desulfurization activity and decreased catalyst life. Ultimately, the catalyst cannot be regenerated.

WHERE DOES SILICON COME FROM?
Recycled Toluene- In 2007 there was an incident in the United Kingdom (UK) where toluene used to wash silicon chips and other electronic components during manufacturing was recycled and used in gasoline blending to increase gasoline octane. This resulted in silicon contaminated gasoline at the pump and thousands of automobile failures.
- In 2009 there was an incident in the United States (US) where silicon contamination occurred with the addition of fuel ethanol to gasoline. Again, the result was widespread vehicle failure. In this incident, it is not clear whether the ethanol was recycled from the cosmetics industry, where it may have come in contact with silicon-containing antifoam agents commonly used in cosmetics, or if the ethanol was manufactured using silicon-containing antifoam agents.
In both the UK and US incidents, silicon was introduced accidentally as a contaminant in a gasoline blending component. As these examples demonstrate, multiple points along the supply chain are vulnerable to silicon contamination. Unfortunately, it is unknown as to how much silicon it takes to contaminate fuel. The contaminated fuel ethanol in the US incident contained more than 100 parts per million (ppm) of silicon, leading to more than 10 ppm of silicon found in the gasoline-ethanol blend samples taken from the affected gasoline stations.
Antifoam agents
- Silicon contamination due to antifoam agents is not exclusive to the ethanol industry. Antifoam agents are often used to minimize foaming at the coker and they also may be used in crude oil extraction. These antifoam agents form breakdown products that end up in the naphtha fraction either through crude distillation or cracking in the coker.
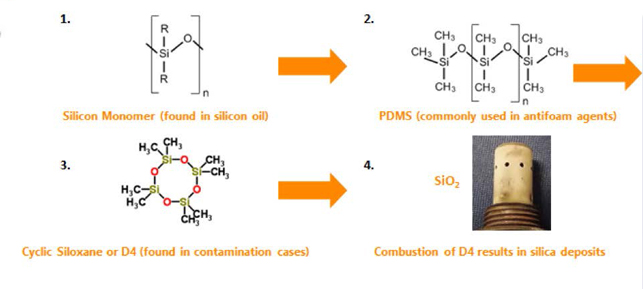
Silicon oils are excellent antifoam agents. Known as polymerized siloxanes, silicon oils can simply be described as a chain of alternating silicon and oxygen atoms that form a backbone to which organic side chains (R) attach. The siloxane monomer unit or “building block,” depicted in the following figure, forms the backbone of the polymerized siloxane, where n is the number of monomer units in the siloxane polymer.
Polydimethylsiloxane (PDMS), commonly used in antifoam agents, breaks down into cyclic siloxanes. These compounds are what is primarily found in contaminated gasoline and ethanol. Octamethylcyclotetrasiloxane (also known as D4) is one of the predominant cyclic siloxanes found in these contamination issues. Combustion of D4 (see graphic below) in an automotive engine forms silicon dioxide (SiO2), or silica, which forms a hard, white deposit on spark plugs, oxygen sensors, and other components.
TESTING FOR SILICON
ASTM D7757 is the only ASTM-approved test method for the determination of silicon in gasoline and ethanol. The scope of the method is for 3-100 mg/kg (weight ppm) of silicon in the following fuels:
- Naphthas
- Gasoline
- RFG
- Ethanol
- Ethanol-fuel blends
- Toluene
D7757 is an MWDXRF method which requires minimal sample preparation, the measurement is non-destructive, and typical analysis time is five to ten minutes per sample.
Method calibration is a weighted linear regression, based on five calibration standards. To account for the matrix differences in gasoline, ethanol, and gasoline ethanol blends, it is recommended to set up an isooctane calibration curve and/or an ethanol calibration curve (if needed) and use correction factors to account for matrix differences. Matrix correction factors are provided in the test method.
INTERLABORATORY STUDY
An Interlaboratory Study (ILS) was performed to determine test method precision of ASTM D7757 using XOS’ Signal analyzers. This study included six laboratories with participants from petroleum refineries and research labs, a third-party test lab, a government contractor, and an automobile manufacturer. The participants analyzed 26 samples in duplicate, and the sample set was comprised of gasoline, gasoline with 10% ethanol, naphtha, toluene, E85, and E100. Table 1 shows the calculated values.
| Table 1: Precision Values, All Sample Types | ||
|---|---|---|
| Si mg/kg (ppm) |
Repeatability (r) mg/kg (ppm) Eq. 1 values |
Reproducibility (R) mg/kg (ppm) Eq. 2 values |
| 3.0 | 1.0 | 1.9 |
| 5.0 | 1.3 | 2.5 |
| 10.0 | 2.0 | 3.7 |
| 25.0 | 3.2 | 6.1 |
| 50.0 | 4.7 | 9.0 |
| 100.0 | 6.9 | 13.1 |
The pooled limit of quantification (PLOQ) was estimated to be 3 mg/kg. The calculated values in Table 1 are derived from the following equations:
Equation 1:
- Repeatability (r) = 0.5582 * X0.5471
Equation 2:
- Reproducibility (R) = 1.0535 * X0.5471
CONCLUSION
With silicon being of increased importance to refiners, the need for precise and timely analysis is critical. As shown in the ILS results, Signal delivers good repeatability and reproducibility for silicon in gasoline and related materials, and is a viable solution with an approved ASTM method to avoid possible naptha hydrotreater catalyst poisoning.
REFERENCES
- ASTM D4814-19, Standard Specification for Automotive Spark-Ignition Engine Fuel, ASTM International, West Conshohocken, PA, 2019, www.astm.org
- ASTM D4806-19, Standard Specification for Denatured Fuel Ethanol for Blending with Gasolines for Use as Automotive Spark-Ignition Engine Fuel, ASTM International, West Conshohocken, PA, 2019, www.astm.org
- ASTM D5798-19a, Standard Specification for Ethanol Fuel Blends for Flexible-Fuel Automotive Spark-Ignition Engines, ASTM International, West Conshohocken, PA, 2019, www.astm.org
- ASTM D5797-18, Standard Specification for Methanol Fuel Blends (M51–M85) for Methanol-Capable Automotive Spark-Ignition Engines, ASTM International, West Conshohocken, PA, 2018, www.astm.org
- ASTM D5983-18, Standard Specification for Methyl Tertiary-Butyl Ether (MTBE) for Blending With Gasolines for Use as Automotive Spark-Ignition Engine Fuel, ASTM International, West Conshohocken, PA, 2018, www.astm.org
For more information about the test method or the interlaboratory study, visit astm.org.
| Table 2: Precision Typical repeatability (r) and reproducibility (R) values in gasoline, at 95% confidence. 600 s measurement time. |
||
|---|---|---|
| Silicon Concentration (ppm) | r | R |
| 2 | 0.4 | 0.7 |
| 5 | 0.5 | 0.8 |
| 8 | 0.6 | 1.0 |
| 15 | 0.8 | 1.4 |
| 100 | 2 | 4 |
| 500 | 5 | 10 |

SILICON ANALYSIS IN PETROLEUM & BIOFUELS

From gasoline to ethanal and toluene, Signal delivers total silicon analysis. Powered by MWDXRF, Signal is a robust analysis solution for demanding petroleum and industrial environment. This analyzer delivers an LOD as low as 0.65 ppm and does not require conversion gasses, heating elements, quartz tubes or columns. See Table 2 for typical repeatability and reproducibility values for silicon in gasoline.
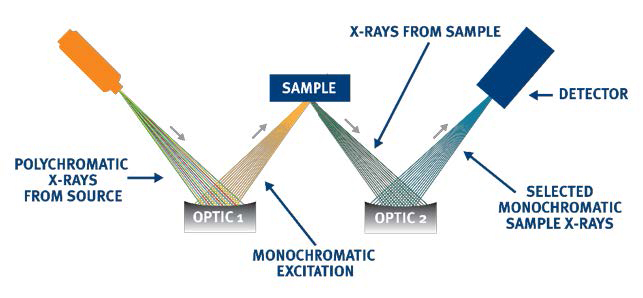
TRUSTED PRECISION WITH MWDXRF
Monochromatic Wavelength Dispersive X-ray Fluorescence (MWDXRF) utilizes state-of-the-art focusing and monochromating optics to increase excitation intensity and dramatically improve signalto-background over high power traditional WDXRF instruments. This enables significantly improved detection limits and precision, and a reduced sensitivity to matrix effects. A monochromatic and focused primary beam excites the sample and secondary characteristic fluorescence X-rays are emitted from the sample. A second monochromating optic selects the silicon characteristic X-rays and directs these X-rays to the detector. MWDXRF is a direct measurement technique and does not require consumable gasses or sample conversion.
Visit xos.com/signal to learn more

