- Analyzers
- Optics & Sources
- Technologies
- Support
- About
XOS - US
Choose your country or region:
Get eNewsletter 1.518.880.1500 Service
Challenge Accepted: Solving the XRF Matrix Mismatch Issue
Leslie McHenry
Challenge Accepted: Solving the XRF Matrix Mismatch Issue
Leslie McHenry
Background
One of the main advantages of XRF analysis is its simplicity and ease of use. In many cases, sample prep is minimal; just put the sample into the cup and measure. In minutes, the measurement result is complete, and cleanup is especially simple when using disposable cups. When finished, no flushing or other complicated procedures are needed to prepare the analyzer for the next analysis. Additionally, many XRF analyzers have autosamplers, allowing the operator to set the analyzer up and then walk away, providing unmatched convenience.
However, as with any analytical technique, XRF has its challenges. In this paper, we will focus on its dependence on the matrix, specifically how to breach calibration standards/sample mismatch. If not corrected, this mismatch can lead to measurement result bias. While there are multiple ways to handle this challenge, let’s demystify some common corrections used on monochromatic wavelength dispersive X-ray fluorescence (MWDXRF) and monochromatic energy dispersive X-ray fluorescence (EDXRF) analyzers, and look at some tips and tricks for performing these corrections.
Common XRF Challenges:
- C/H ratio differences for petroleum and hydrocarbon samples
- The high-low sulfur-chlorine relationship
- Correcting for low bias in biofuels, bio-feedstocks, and other oxygenated fuels
- Other matrix correction challenges, such as catalyst, coal, and water

Get in Touch
Challenge 1: C/H ratio differences for petroleum and hydrocarbon samples
With the advent of the U.S. Environmental Protection Agency’s (EPA) streamlining gasoline regulations, users were faced with a choice when certifying sulfur in gasoline–use referee procedure ASTM D2622 (sulfur by WDXRF) specified in EPA 40 CFR part 1090.1360 or run the Performance Based Measurement System (PBMS) testing to use their preferred method. Then, many non-D2622 XRF users discovered that gasoline biased high on their mineral oil calibrations due to a carbon to hydrogen (C/H) ratio difference, causing difficulty in passing the PBMS accuracy testing. In this case, the easiest solution was to switch to gasoline or synthetic gasoline calibrants using an isooctane-toluene blend, approximating the number of aromatics present in their finished gasoline samples (see ASTM D7039 sulfur by MWDXRF section 5.4.1).
For this application, this is an optimal solution as there are multiple calibration vendors available who can provide natural or synthetic sulfur in gasoline standards if the user chooses not to make their own. Whichever route is chosen, ensure a good blank for optimal performance at low concentrations. For more information on improving gasoline calibrations, see Chasing the Bias – Gasoline Matrix Matching to Improve ASTM D2622 Sulfur Measurement Accuracy. (Although this paper looks at D2622 calibrations in detail, many tips apply to other methods.)
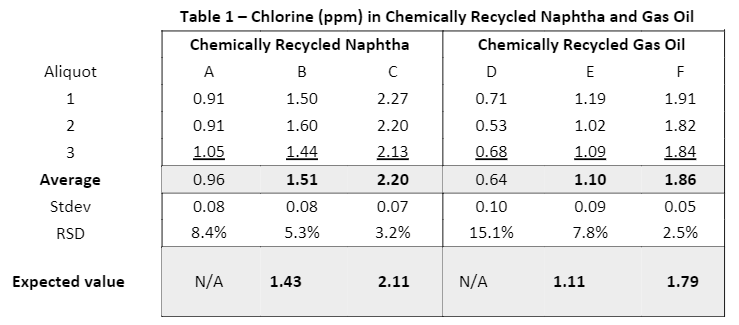
Sometimes, an exact calibration standards/sample match may not be needed. This is especially true at very low sample concentrations. Let’s look at the example given in the article Clora 2XP: Accurate Chlorine Measurement for Advanced Plastic Recycling (xos.com), where Clora 2XP was calibrated with mineral oil standards using a modified ASTM D7536 methodology and then measured doped and undoped chemically recycled naphtha and gas oil samples. Based on the aforementioned gasoline application, the chlorine in naphtha measurement is expected to bias high, and technically, it does; however, C/H bias scales with concentration. This means that sample B, expected to have 1.43 ppm chlorine, measured an average of 1.51 ppm. Sample C, expected to have 2.11 ppm chlorine, measured an average of 2.20 ppm. See Table 1. Both samples biased high, but the difference was not significant enough to prevent the customer from purchasing and adopting Clora 2XP to monitor chlorine in plastic recycling. We’ll return to this example in the next section, but before we do, let’s spend a moment on mathematical C/H ratio correction.
Petra uses a mathematical solution for resolving C/H ratio differences between the sample and calibration by using information from the deconvolution of the elastic and inelastic X-ray scattering within the sample spectrum. Though the principle is complex, it results in an automatic C/H ratio correction that does not require multiple calibration curves for different hydrocarbon types. In the paper Automatic Matrix Correction for C/H Ratio in Sulfur Analysis Using ASTM D4294 and ISO 8754, Petra 4294 used a mineral oil calibration (5.6 C/H ratio) combined with automatic C/H ratio correction to demonstrate accurate sulfur in xylene (9.6 C/H ratio) and crude oil (unknown C/H ratio) measurements with minimal bias (<1%).
Challenge 2: The high-low sulfur-chlorine relationship
Section 6 of ASTM D4929 for Determination of Organic Chloride Content in Crude Oil lists interferences for procedures A, B, and C. Section 6.3 specifies interferences for Procedure C, XRF. In addition to mentioning C/H ratio correction through matrix matching, it also talks about sulfur interference on chlorine. High sulfur absorbs chlorine Kα sample fluorescence, leading to low chlorine results. If uncorrected for, it may lead to inaccurate chlorine reporting and unchecked corrosion, which is a costly consequence. D4929 specifies dilution, matrix matching, and sulfur correction factors to mitigate the effects of sulfur on chlorine. Dilution is probably the poorest option for correction, as it lowers the amount of chlorine in the specimen (which is typically already low in the sample to start) and magnifies measurement uncertainty when the dilution factor is accounted for. Matrix matching is not an ideal solution either, as sulfur levels are often inconsistent in the sample. The best solution for this application is the use of sulfur correction factors.
As D4929 states, these are typically applied in the software and use one of the following forms:
- Manual input of sulfur concentration followed by automatic correction
- Direct measurement of sulfur followed by automatic correction
- Correction by use of Compton scattering
- Correction by applying fundamental parameters
Sindie +Cl and Clora product lines correct for sulfur either through manual input of sulfur (Clora) or direct measurement of sulfur (Sindie +Cl and Clora 2XP), followed by correction.
Note that sulfur correction is not only for crude oil. It is also applicable to any high sulfur - low chlorine fuel, such as VGO, coker residual, and other refinery intermediates. Returning to our advanced plastic recycling example from the previous section, both the naphtha and gas oil exhibited high sulfur counts, requiring the use of Clora 2XP’s automatic sulfur correction. The customer chose Clora 2XP over Clora for this application for its automatic sulfur correction. This way, the technician does not have to do an additional sulfur measurement (to get the sulfur value for manual input on Clora) to get an accurate chlorine result, thus saving time and reducing inaccuracies due to manual input errors.
Product Highlight: Clora 2XP
Improve X-ray analysis performance and capability
A polycapillary optic captures a large solid angle of X-rays from an X-ray source and redirects them to a micron-sized focal spot or a highly collimated beam. The X-ray intensity achieved with such optics is a few orders of magnitude higher than that obtained with conventional pinhole collimators, contributing to the significantly improved X-ray analysis performance in detection sensitivity, spatial resolution, measurement speed, and precision. XOS optics are widely used in commercial instruments and customized X-ray analysis systems for various industrial and research applications in the fields such as microelectronics, semiconductor manufacturing, pharma, and life sciences.

Challenge 3: Oxygen got you down? Correct for low bias in oxygenated fuels
Another calibration-sample mismatch issue is seen in oxygenated fuels, such as biodiesel, bio-oils, bio-marine fuel, bio-feedstocks, and ethanol. Oxygen absorbs X-rays, especially when measuring light elements such as sulfur and chlorine. If not corrected for, oxygen leads to low measurement bias, inaccurate reporting, potential fines or contract disputes, and corrosion issues. Oxygen interference is a common issue recognized by many sulfur XRF methods, including ASTM D2622, D4294, D7039, ISO 20884, ISO 8754, ISO 13032, and EN 16997. This problem is not limited to sulfur alone. For example, chlorine, calcium, phosphorus, and silicon are also affected by oxygen absorbance. However, some analyzers, including XOS analyzers, correct low bias due to oxygen can by matrix matching, manual correction, or automatic correction depending on analyzer and application type.
For XOS MWDXRF analyzers such as Sindie, Clora, Phoebe (P), and Signal (Si), oxygen correction is typically handled through matrix matching or use of correction factors that are manually applied after measurement. For sulfur measurement, where commercial standards are readily available for various natural or synthetic fuel substitutes, matrix matching is commonly employed, especially when the oxygen content is consistent in the sample. This becomes more difficult if the oxygen content is not consistent, something commonly seen in bio-feedstocks. In this case, rather than maintaining multiple calibration curves for different oxygen content, use of a single calibration curve with multiple correction factors (depending on oxygen content) is preferred.
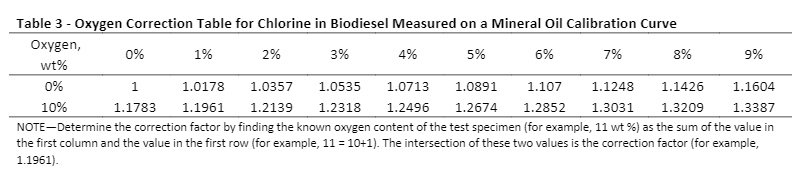
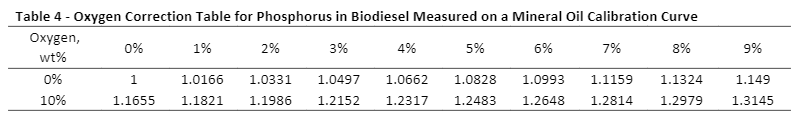
Some of these tables can be found in the standard test methods. ASTM D7039 (S by MWDXRF) has three oxygen correction tables, ASTM D7757 (Si by MWDXRF) has two oxygen correction tables, and EN 16997 (S in E85 by WDXRF) has one correction table to correct for oxygen interference on the element of interest in different sample types (see Table 2). Note that these tables are specific for monochromatic excitation and are based on the calculations in Annex A1 of the corresponding ASTM method (the EN method calculations are based on Annex A1 in D7039). For oxygen correction tables for chlorine and phosphorus measurements, see Tables 3 and 4, respectively. To apply these correction factors, first set up a calibration with the calibration matrix specified in each table. After that, unknown samples can be measured on this calibration, and the correction factor (corresponding to the known amount of oxygen in the sample) is multiplied by the measured result to obtain the oxygen-corrected value.
Note that this type of bias also scales with concentration and is often not a concern for very low concentration samples (<1 ppm), so much so that correction is often ignored. This becomes a problem, however, once concentration increases. A good example of this is seen in Precision Comparison Between UVF and MWDXRF for Biodiesel Samples, which looks at the ASTM B100 Proficiency Testing Program (PTP), comparing D5453 (sulfur by Ultraviolet Fluorescence) with ASTM D7039. Use matrix matching or correction factors to correct for low bias.
On the EDXRF side, Petra 4294 and Petra MAX have two options for biofuels and oxygenated hydrocarbons—matrix matching and autocorrection. In Advanced Bio Marine Fuel Testing – No More Oxygen Worries, this paper details how to set up both of these correction methods on Petra, and how they compare. Matrix matching is the clear accuracy winner, though autocorrection offers more flexibility when dealing with samples with varying oxygen content. Also, although this paper focuses on sulfur testing in bio-marine fuels, these same correction methods work for other elements on Petra MAX, such as phosphorus and chlorine, when in high enough concentrations to be measured on Petra MAX.
Challenge 4: Other matrix mismatch issues
The previous sections dealt with some of the most common matrix challenges seen by XOS customers, and some tips for correcting for them. This section deals with other less common but equally interesting applications. The list below describes how XOS MWDXRF and monochromatic EDXRF analyzers compensate for these matrix mismatch issues:
- Petra MAX’s larger spot size makes it preferred over Clora for monitoring chlorine in powdered alumina-supported catalyst samples, according to UOP 979. While the default fundamental parameters (FP) calibration can get approximate (or screening) results, use matrix matching for best results. See A Reliable Solution to Measure Chlorine in Catalyst by UOP 979 for details.
- In Fast and Precise Elemental Screening in Petroleum Coke and Coal, matrix-matched calibrations are used for sulfur in coal and coke, and default FP is used for Ca, V, Mn, Fe, and Ni.
- In Challenge 3, we discussed oxygen in terms of biofuels, but what about measuring elements in water itself? Use the default FP calibration on Petra MAX for screening, or matrix match for more accurate results. Read Detailed How to Measure Chlorides in Crude Oil by ASTM D4929C and Water Extraction to learn more about crude oil water extraction and how a correction factor of 2.5 can be used on Clora and Clora 2XP with a mineral oil calibration to measure chlorine in water samples.
Conclusion
All elemental analysis techniques have their strengths and challenges, and XRF is non-destructive, reliable, and easy to use. Its main challenge lies in matrix dependence, or knowing how to correct for differences between calibration standards and samples. In summary, if the bias is significant, it must be corrected for. A primary theme for MWDXRF analyzers, such as Sindie and Clora, has been matrix matching or correction factors. This is due to the monochromatic excitation, which simplifies matrix correction due to the removal of background noise. For Petra monochromatic EDXRF, depending on the application, it may use algorithms in the form of its fundamental parameters calibration, matrix matching, or a combination of both to combat the matrix mismatch issue.
Have a question about how to handle a unique matrix not covered in this paper? Schedule an expert consultation.
About the Author

Leslie McHenry is Applications Supervisor at XOS. For over a decade, Leslie has been helping leading laboratories and petroleum refineries around the world meet their testing needs. Leslie is an active member of the ASTM International community and has co-authored several ASTM XRF test methods. Prior to joining XOS, Leslie worked as a Petroleum Chemist at a North American petroleum refinery.
If you'd like to get in touch with Leslie or one of our other experts, click here.
15 Tech Valley Drive | East Greenbush, NY 12061
Phone: (US) 1.518.880.1500 | Fax: (US) 1.518.880.1510
info@xos.com
Phone: (US) 1.518.880.1500 | Fax: (US) 1.518.880.1510
info@xos.com

